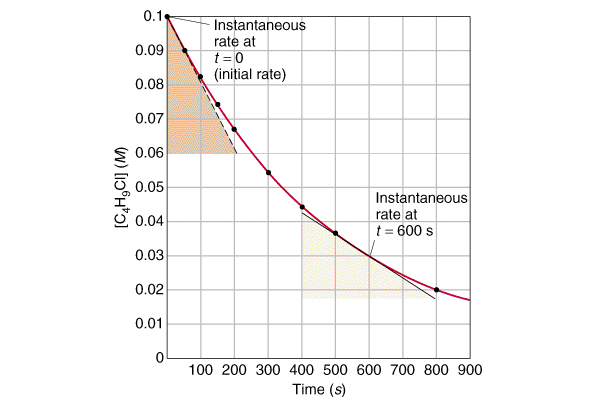
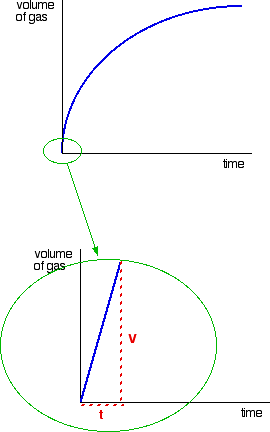
The method of initial rates and the graphical method. Chemists therefore tend to describe reactions by their initial rate which refers to the rate of reaction during the first few seconds or minutes.
how to calculate the initial rate of a reaction
how to calculate the initial rate of a reaction is a summary of the best information with HD images sourced from all the most popular websites in the world. You can access all contents by clicking the download button. If want a higher resolution you can find it on Google Images.
Note: Copyright of all images in how to calculate the initial rate of a reaction content depends on the source site. We hope you do not use it for commercial purposes.
A average rate of reaction b rate of reaction at a given time the average rate of reaction is the average value of the rate of reaction within a specified.
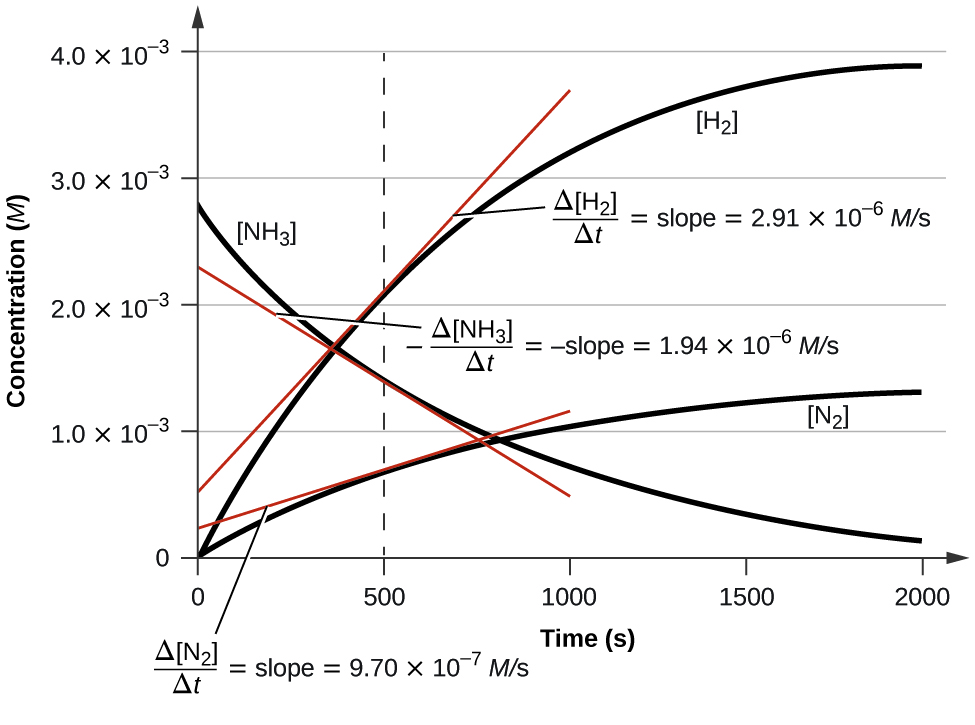
How to calculate the initial rate of a reaction. Finding the rate law using the method of initial rates. Finding the initial rate of a reaction russ cornelius. Initial rates method for determining reaction order rate laws rate constant k chemical kinetics duration.
The initial rate of a reaction is the instantaneous rate at the start of the reaction ie when t 0. The initial rate is equal to the negative of the slope of the curve of reactant concentration versus time at t 0. So 098 100 and this is all over the final time minus the initial time so this is over 2 0.
002 here over 2 and that would give us a negative rate of reaction but in chemistry the rate of reaction is defined as a positive quantity. Calculate average reaction rates given. Methods to measure the rate of reaction the rate of reaction can be measured in two ways.
Chemistry with ken 21968 views. Determining the initial rate from a plot of concentration versus time. Now this would give us 002.
Two methods are commonly used in the experimental determination of the rate law. The initial rate is equal to the negative of the slope of the curve of reactant concentration versus time at t 0. Determining rate by tangent.
The initial rate of a reaction is the instantaneous rate at the start of the reaction ie when t 0. As a reaction proceeds the rate tends to decrease because the chance of a collision between reactants becomes progressively lower. How do you calculate the reaction rate.
So you need to distinguish between the instantaneous reaction rate that is the rate for a given instant and the average rate which determines the rate over the course of the reaction. The rate law of a chemical reaction is a mathematical equation that describes how the reaction rate depends upon the concentration of each reactant. The idea is that you want to find the instantaneous rate of change rise over runat the initial value time 0.
But since this gives 40 cant be done just use a close number to the initial value to get a good approximation of the slope at the value. The organic chemistry tutor 234404 views. Kac253 rates ii.
So we need a negative sign. The rate of a reaction can change over time. As a reactant is used up for example its rate typically decreases.
 Finding The Initial Rate Of A Reaction Youtube
Finding The Initial Rate Of A Reaction Youtube
 How Do You Calculate The Initial Rate Of Reaction The Student Room
How Do You Calculate The Initial Rate Of Reaction The Student Room
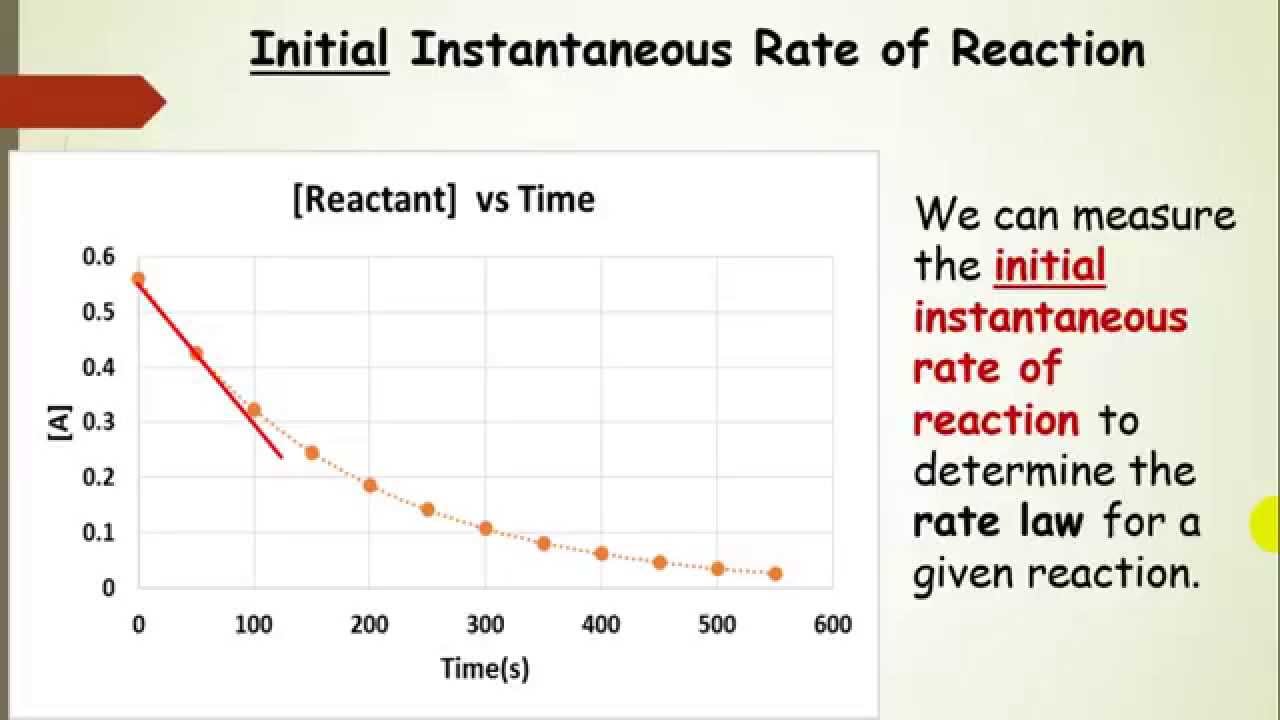 Finding The Rate Law Using Method Of Initial Rates Experiments
Finding The Rate Law Using Method Of Initial Rates Experiments
Meaning Definition Of Rate Of Reaction Slow Fast Apparatus
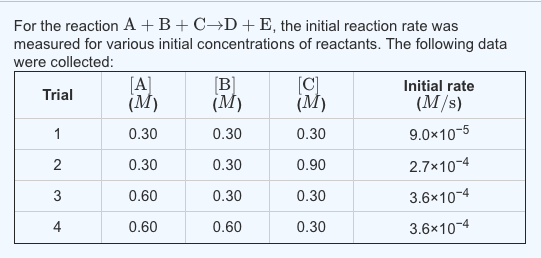 Solved Calculate The Initial Rate For The Formation Of C
Solved Calculate The Initial Rate For The Formation Of C
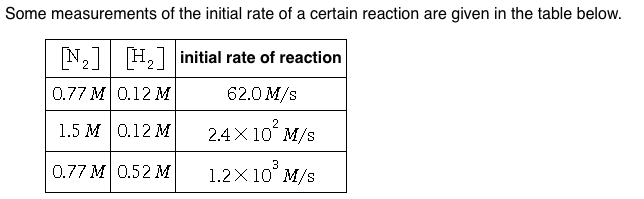 Solved Some Measurements Of The Initial Rate Of A Certain
Solved Some Measurements Of The Initial Rate Of A Certain
Reaction Rates Introductory Chemistry
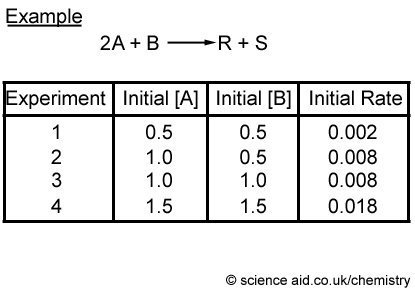 Kinetics Rate Of Reaction Order Of Equation Scienceaid
Kinetics Rate Of Reaction Order Of Equation Scienceaid
 What Is Instantaneous Rate Of Reaction And How We Can Determine
What Is Instantaneous Rate Of Reaction And How We Can Determine
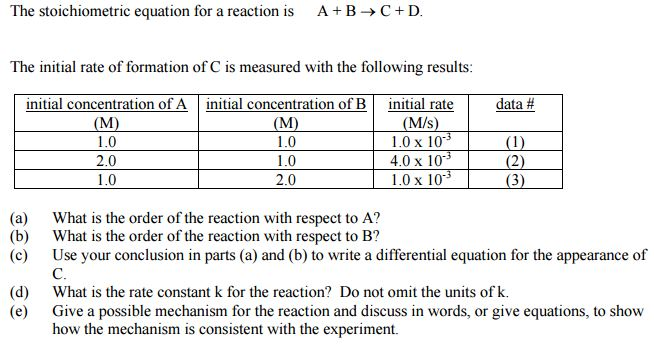 Solved The Stoichiometric Equation For A Reaction Is A
Solved The Stoichiometric Equation For A Reaction Is A